Calprotectin
What is Calprotectin
Calprotectin is a 36 kDa heterodimer belonging to the S100 protein family, produced by polymorphonuclear cells such as monocytes and macrophages. It is characterized by pro-apoptotic, bacteriostatic, and antifungal properties. As a member of the S100 protein group, it is involved in the activation and modulation of inflammatory processes: in fact, it is secreted at the site of injury where the inflammatory response begins, and it exerts its action by sequestering metal ions.
Thanks to its biological properties and its ability to resist degradation, calprotectin has become an important marker of inflammation in recent years. It can be easily extracted and quantified from various biological matrices, including stool, serum, plasma, saliva, cerebrospinal fluid, synovial fluid, and urine.
Why detect it?
Inflammatory states originate from an excessive immune reaction that leads to reduced organ functionality. This condition can be linked to various circumstances and disorders. The involvement of calprotectin in the inflammatory process has been widely demonstrated, and recently its role in the pathogenesis, diagnosis, and monitoring of certain inflammatory rheumatic diseases, as well as other conditions such as obesity and colorectal cancer, has come to light.
Measuring fecal calprotectin is particularly crucial for differentiating between chronic inflammatory bowel diseases (IBD) 1,2,3,4 —such as Crohn’s disease and ulcerative colitis—and irritable bowel syndrome (IBS). These two conditions share very similar symptoms. Fecal calprotectin testing is a quick, non-invasive1 examination that helps distinguish patients with significant inflammatory activity associated with serious pathologies (IBD) from those with functional intestinal disorders (IBS), who represent 35-50% of individuals undergoing gastroenterological examinations.
Calprotectin determination has also found important applications in pediatric and neonatal settings 5,6,7 , as elevated levels of fecal calprotectin are observed in pediatric patients with IBD. In these cases, both the sensitivity and specificity of calprotectin measurement are more reliable than other biochemical parameters typically used to detect intestinal inflammation.
However, pediatric cut-off values are higher than those found in adults; it is important to remember that a newborn’s intestinal function is not yet fully developed, and the introduction of breast milk or other foods in the first days of life triggers an inflammatory response, resulting in extremely high levels of calprotectin. Consequently, very low or absent calprotectin in newborns could indicate other intestinal conditions requiring further investigation. In normal physiological conditions, calprotectin levels generally decrease as the child grows, corresponding to the normalization of the mucosal layer.
References
- F. Khaki-Khatibi et al. Calprotectin in inflammatory bowel disease. Clinica Chimica Acta 510 (2020) 556–565.
- B Alibrahim et al. Fecal calprotectin use in inflammatory bowel disease and beyond: A mini-review. Can J Gastroenterol Hepatol Vol 29 No 3 April 2015.
- Kapel et al. Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases. American College of Gastroenterology, Clinical and Translational Gastroenterology 2023.
- T. Sipponen, K-L Kolho. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. 2015 Jan;50(1):74-80. doi: 10.3109/00365521.2014.987809.
- R. Berni Canani et al. Fecal calprotectin is a useful diagnostic tool in pediatric gastroenterology. ITAL J PEDIATR 2005;31:89-94.
- F. Campeotto et al. Fecal Calprotectin: Cutoff Values for Identifying Intestinal Distress in Preterm Infants. J Pediatr Gastroenterol Nutr, Vol. 48, No. 4, April 2009.
- M. Roca et al. Fecal calprotectin in healthy children aged 4–16 years. Nature Reesearch, Scientific Reports | (2020) 10:20565 | https://doi.org/10.1038/s41598-020-77625-7.
FAQ
-
These are the symptoms related to Inflammatory Bowel Diseases (IBD) such as ulcerative colitis and Crohn’s disease.
The most common include:
- Recurrent diarrhea (possibly abundant) with or without bleeding
- Rectal mucus discharge
- Diarrhea often accompanied by severe abdominal pain (which can sometimes be mistaken for appendicitis.
- In some cases, fever and weight loss caused by reduced appetite or malabsorption (weight loss of 10-15 kg in a short period can occur)
-
Measuring fecal calprotectin provides significant benefits in assessing the local degree of intestinal inflammation. In patients with IBD, calprotectin levels are typically very high, whereas in patients with Irritable Bowel Syndrome (IBS), calprotectin levels are above those of healthy subjects but significantly lower than those found in patients with active disease.
-
The fecal concentration of calprotectin is expressed in milligrams of calprotectin per kilogram of stool (mg/kg). However, some scientific publications may express this concentration in micrograms per gram (µg/g).
-
Taking large amounts of non-steroidal anti-inflammatory drugs (NSAIDs) can trigger inflammatory processes that may increase calprotectin levels. It is therefore important to note any use of these medications.
-
The stool sample is easily collected with the EasyCal medical device, designed for pre-analytical processing of stool samples.
Each EasyCal variant is linked to a specific analytical kit and allows both the extraction and storage of one or more diagnostic markers. Please contact us to determine the best solution for your laboratory needs.
-
Yes, the stool sample can be collected at home using the ready-to-use EasyCal device, which contains the extraction buffer for the required marker. The sample can then be returned to the laboratory for analysis.
-
Yes, stool samples of various consistencies can be analyzed. To help classify the stool sample correctly, it is recommended to refer to the “Bristol Stool Scale.”
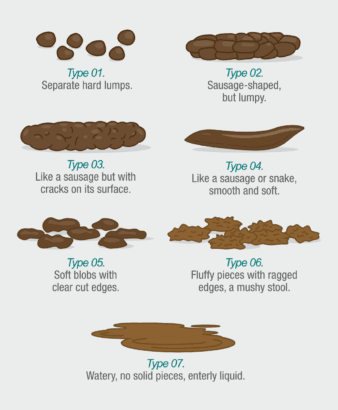
For self-collection (based on the Bristol Stool Scale), only stool samples categorized as types 2 through 6 should be used. We recommend avoiding self-collection for samples classified as type 1 (very hard) or type 7 (very liquid) on the Bristol Stool Scale.
For professional use, you can collect 24 µl of liquid stool with a laboratory pipette and then proceed with extraction using EasyCal.
-
In the laboratory, stool samples should be stored at 2-8°C for up to 2 days before testing. If not tested immediately, they can be frozen at -20°C for up to one year. If analyzing frozen samples, avoid more than two freeze/thaw cycles.
Samples collected and extracted with the EasyCal device can be analyzed immediately or stored under the following conditions:
- Up to 14 days at 2-8°C
- Up to 3 days at room temperature
-
Because there is no quantified international standard, it is difficult to normalize data obtained through different technologies and tests. Consequently, some degree of variability in the results can be observed.

